ISO 9001 Risk Management Procedure Template
QMS 9001 Risk Procedure is a process-related document that outlines how to identify, analyze, and respond to risks associated with the organization's quality management system. It helps management ensure that the quality management system operates effectively and efficiently while protecting employees, customers, and other interested parties. The procedure also helps ensure that risks are minimized and that potential impacts are identified and addressed in a timely manner.
There are four main sections to risk procedure. These sections include:
- Introduction
- Definition of risk
- Risk identification process
- Risk analysis process
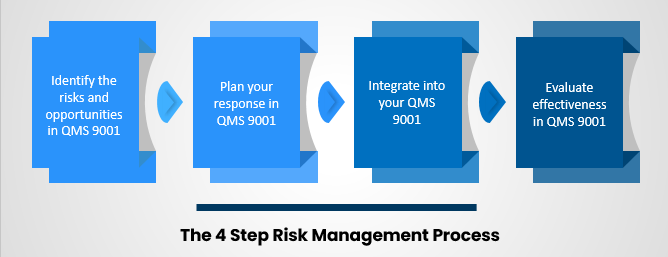
The 4 Step ISO 9001 Risk Management Process
1. Identify The Risks And Opportunities In QMS 9001: QMS 9001 is an international quality management system standard. It provides a framework for organizations to ensure that their products and services meet customer and regulatory requirements. There are many benefits to implementing a QMS 9001, including reducing waste, improving efficiency, and enhancing customer satisfaction. There are four main components of the QMS 9001 standard: quality policy, quality objectives, resource management, and product realization.
The quality policy establishes the framework for an organization’s commitment to quality. Quality objectives define what an organization hopes to achieve through its QMS 9001 implementation. Resource management ensures that resources are available when needed and used in an effective manner. Product realization includes all activities necessary to produce a product or service from start to finish.
2. Plan Your Response In QMS 9001: The ISO 9001 Quality Management System (QMS) requires the organization to have a process for planning and controlling its activities. This process, known as risk management, is designed to identify potential risks and opportunities associated with the organization's products, services, processes, and projects. Risk management helps prevent or reduce the impact of risks on objectives and facilitates decision-making by providing a structured approach for assessing and responding to risk.
Risks can be classified as either:
- Risks that could occur and have an impact on the organization's ability to achieve its objectives.
- Risks that have already occurred and have had an impact on the organization.

- Understand the requirements of ISO 9001:2015. This may seem like a no-brainer, but it’s important that you have a good understanding of the Standards requirements before you start your journey towards certification.
- Engage employees in the process. One of the best ways to ensure buy-in from employees is to get them involved in the process from the beginning. Consider holding a company-wide meeting to let everyone know about your plans to implement ISO 9001:2015 and what it will mean for them.
- Determine which requirements are applicable to your organization. Not all of the requirements in ISO 9001:2015 will be applicable to every organization. Determine which ones make sense for your business and focus on those first.
- Implement a document control system. A document control system will help you keep track of all the documents and procedures related to your QMS. This will be especially important once you achieve certification and need to maintain compliance with the standard’s requirements.
- Train employees on the new QMS procedures. Once you’ve developed new procedures, it’s important that you train all employees on how to follow them correctly. Consider holding regular training sessions or creating informational documents explaining processes in more detail.
4. Evaluate Effectiveness In QMS 9001: ISO 9001:2015 is the most recent version of the Quality Management System (QMS) standard. It places a stronger focus on risk-based thinking and organizational improvement. The standard requires that organizations demonstrate the effectiveness of their QMS through regular performance evaluation. This can be accomplished through management review, internal audits, and supplier audits.
ISO 9001:2015 is based on the Plan-Do-Check-Act (PDCA) quality management cycle. The PDCA cycle consists of four steps: plan, do, check, and act. The first step is to identify the goals and objectives that need to be met. The second step is to implement the plans and procedures necessary to achieve those goals. The third step is to check the results of the implementation against the objectives. The fourth step is to take corrective action if necessary.
The Benefits Of QMS 9001 Risk Procedure:
QMS 9001 Risk Procedure sets out how the organization can identify, assess and control risks related to quality. It also includes steps for monitoring risk performance and communicating any changes or incidents. Benefits of having a documented QMS 9001 Risk Procedure can include:
- Fewer product defects/returns.
- Increased customer satisfaction.
- Reduced costs associated with product recalls and corrective actions.
- Improved employee morale and productivity.
- Greater regulatory compliance.
- Improved risk management and decision making.
- It helps to improve communication and interaction among departments within an organization.
- It assists in the early identification of potential risks and enables timely corrective action.
- It provides a basis for continual improvement of the effectiveness of the risk management process.
- It facilitates regulatory compliance with statutory and regulatory requirements related to risk management.
Conclusion
QMS 9001 Risk Procedure Template is essential for ensuring that your organization effectively identifies and manages risks. This template provides a structured approach to risk assessment and mitigation, aligning with the requirements of ISO 9001. By utilizing this tool, your organization can enhance its risk management practices and ultimately improve overall performance. Download the QMS 9001 Risk Procedure Template today to streamline your risk management processes and achieve greater organizational success.