ISO 9001 Inspection and Test Records Template
In quality management, inspection and test records are crucial to ensuring compliance with ISO 9001 standards. These records are documented evidence that products and processes have been thoroughly inspected and tested to meet the required quality standards. Effective record-keeping demonstrates a company's commitment to quality control and provides a valuable resource for identifying areas of improvement and ensuring traceability throughout the manufacturing process. In this blog, we will explore the importance of inspection and test records in ISO 9001 and discuss best practices for maintaining accurate and comprehensive records.
Understanding the Purpose and Benefits of Maintaining Accurate Records
In the previous section, we established the significance of inspection and test records in ISO 9001 compliance. Now, let's delve into the purpose and benefits of maintaining accurate records.
The primary purpose of maintaining accurate records is to establish a reliable audit trail. By meticulously documenting inspections and tests conducted throughout the manufacturing process, companies can demonstrate their commitment to meeting quality standards. These records serve as crucial evidence during internal audits and external assessments, providing proof of compliance with ISO 9001 requirements.
Moreover, accurate records act as a valuable resource for identifying areas of improvement within the organization. By reviewing these records, businesses can track trends, identify recurring issues, and implement corrective actions to enhance product quality and optimize processes.
Additionally, maintaining comprehensive records ensures traceability. In the event of product recalls or customer complaints, organizations can easily track the production history, enabling prompt investigation and resolution.
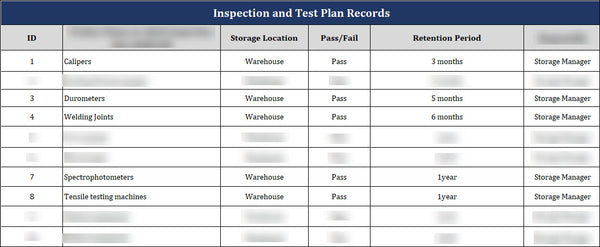
The Key Elements of a Practical Inspection and Test Record
A practical inspection and test record is vital to any quality management system. It allows organizations to document their inspection activities, ensuring that products are checked thoroughly for compliance with applicable standards, regulations, and customer requirements. An inspection and test record should include key elements to make it comprehensive and practical.
- Several key elements should be included in the inspection record when conducting an inspection. The first element is the product name, which identifies the specific product or item that underwent inspection. This is crucial, especially during large-scale inspection processes, as it helps differentiate between various products.
- Another important element in the inspection record is the storage location of the inspected product. This information is essential for traceability and retrieval, especially in complex supply chains or multiple storage locations. Knowing where the product is stored ensures that it can be easily located if any issues arise later.
- The retention period is another crucial element to include in the inspection record. Specifying the duration for which the record should be retained is important. Regulatory requirements, industry standards, and company policies determine this. By specifying the retention period, the inspection record becomes a valuable tool for tracking the product's quality history and addressing any potential recalls or customer complaints.
- Lastly, the responsible party should be mentioned in the inspection record. This ensures accountability and provides a point of contact for any queries or further investigations related to the inspection. It is important to identify the inspector or responsible individual/department to address any concerns or inquiries regarding the inspection. Including these key elements in the inspection record is essential for maintaining product quality, ensuring traceability, and facilitating effective communication and accountability.
A practical inspection and test record should have a clear title, specific storage location, retention period, and responsible party. It should include the date and time of inspection, inspection results and findings, corrective actions taken, the inspector's signature, and any necessary retests or follow-up actions. Including these key elements ensures comprehensive documentation, traceability, and accountability in a quality management system, ultimately improving product quality and customer satisfaction.
Monitoring and Reviewing Inspection and Test Records for Continual Improvement
Now that we understand the purpose and benefits of maintaining accurate inspection and test records, let's explore how to monitor and review these records for continual improvement effectively.
Monitoring and reviewing inspection and test records are essential in the ISO 9001 compliance process. By regularly analysing these records, organizations can identify trends, patterns, and areas that require attention. This information allows businesses to make informed decisions, implement corrective actions, and continuously improve their processes and products.
Companies should establish a structured system to monitor and review inspection and test records. This system should include regular audits of the records, clear guidelines on data analysis, and a mechanism for reporting and addressing any discrepancies identified.
In addition to internal monitoring, seeking external feedback can enhance inspection and test records. Collaborating with industry experts or engaging in benchmarking exercises can provide valuable insights and help organizations stay ahead of the curve.
By prioritizing the monitoring and reviewing inspection and test records, companies can drive continual improvement and remain compliant with ISO 9001 standards.
Conclusion
In conclusion, inspection and test records are crucial in the ISO 9001 compliance process. They provide organizations with valuable insights, allowing them to identify areas for improvement and make informed decisions. By establishing a structured system for monitoring and reviewing these records, businesses can maintain their quality management system and drive continual improvement.
The importance of employee training in ensuring accurate and reliable inspection and test records. Stay tuned to learn how investing in employee training can significantly impact the effectiveness of your quality management system.
Remember, maintaining accurate inspection and test records is not just a requirement for ISO 9001 compliance but also an opportunity for organizations to enhance their processes and products. Join us in the final section as we explore the benefits of employee training in this regard.



